
What
we say:
Magnesium and diatomic oxygen yields magnesium oxide.
Reasoning:
Because we're trying to help learners to learn, it's
probably appropriate to be pretty careful about
spelling out when we're talking about O2 vs O. And the "and" needs to be there
because we need an unambiguous conjunction ("and") that won't be confused with
an ion charge ("plus").
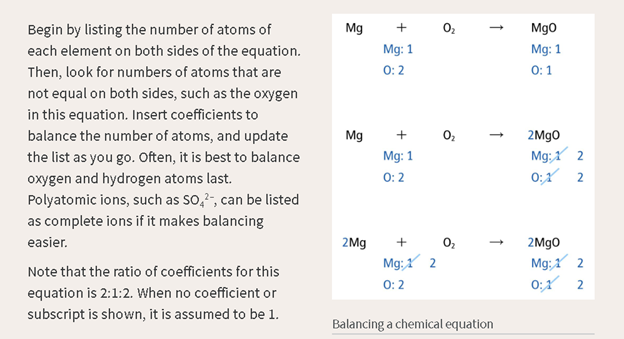
What we say:
The reaction
of magnesium and diatomic oxygen yields magnesium oxide.
Equation 1: Left
side, one magnesium atom, 2 oxygen atoms. Right side, one magnesium atom, one
oxygen atom.
Equation 2: Right
side multiplied by two results in: Left side, one magnesium atom, two oxygen
atoms. Right side, two magnesium atoms, two oxygen atoms.
Etc.
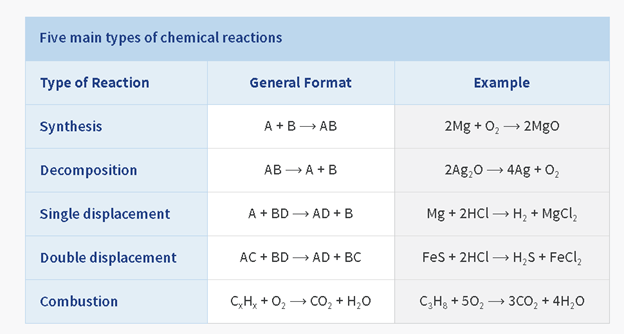
How we read it:
Synthesis
reaction: General format, A and B yields A B. Example, 2 M G and O 2 yields 2 M
G O
Reasoning:
Not reading
formulas here, because we're trying to teach types of reactions. Atomic makeup
of compounds is important.

How we read it:
One unit of
solid copper and 2 units of aqueous silver nitrate yields 2 units of solid
silver and one unit of aqueous silver nitrate.
Reasoning:
We use the
generic term "unit" to emphasize that coefficients in equations can be atoms,
molecules, ions, formula units, moles, or volumes of gas. We also suggest
switching the order, so the states of matter act like adjectives, rather than
being read out in the order it is presented in the notation of the equation.
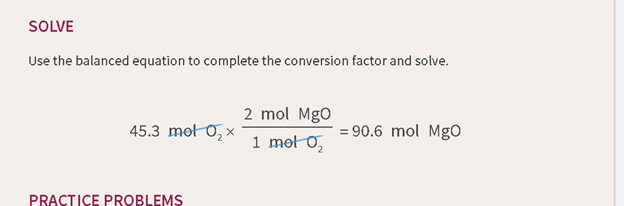

How we read it:
(Top equation)
Forty-five
point three moles of diatomic oxygen times 2 moles of magnesium oxide per 1
mole of oxygen, equals ninety-six point 6 moles of magnesium oxide because
moles of oxygen cancel.
Reasoning:
Here, we want
to emphasize the most important thing first-the result. And then, follow up
with the extras like "what cancels what." Also note, the "per" helps indicate
the relationship that's implied by the "conversion factor" in a way that the word
"over" doesn't really help with.

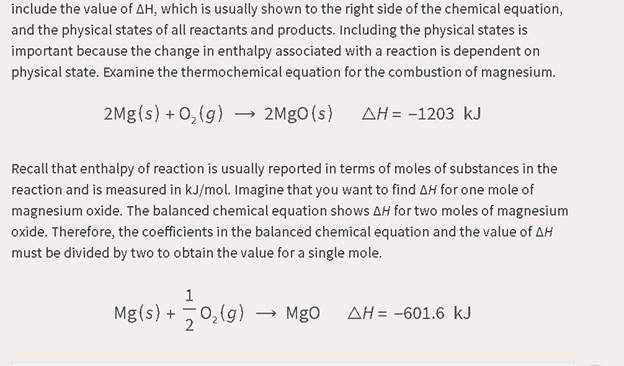
How we read it:
One-half of a
mole of nitrogen gas and one-half of a mole of oxygen gas and ninety point two
nine kilojoules of energy yields one mole of nitrogen oxide gas.
Reasoning:
We use moles
because it really is moles.

How we read it:
Carbon as
graphite and oxygen gas yields carbon dioxide gas, with a change in enthalpy of
negative 393.5 kilojoules. Carbon dioxide gas yields diamond and oxygen gas,
with a change in enthalpy of positive 395.4 kilojoules. When these two
equations are added together, oxygen gas and carbon dioxide gas cancel . The result is graphite yields diamond with a change
in enthalpy of positive 1.9 kilojoules.
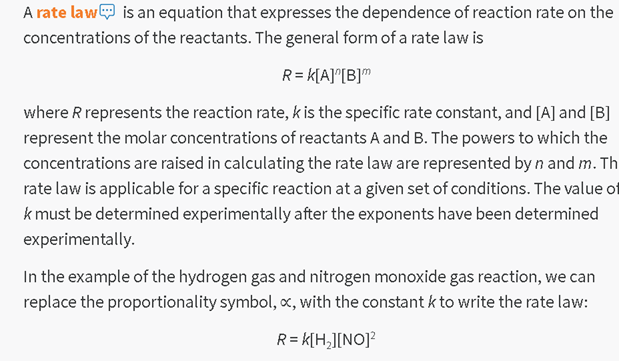
How we read it:
R equals k
times A in brackets to the n power times B in brackets to the m power.
where R
represents the reaction rate, k is the specific rate constant and A in brackets
and B in brackets represent the molar concentrations of reactants A and B....
...
...replace
the proportionality symbol with the constant k to write the rate law:
The rate is
equal to the rate constant times the concentration of hydrogen times the square
of the concentration of the nitrogen oxide.
Reasoning:
In this case,
there is value to letting students know what the notation looks like. Because
the generic equation is immediately followed by the spelled-out-explanation of
what the symbols in the equation mean, that's another reason why we suggest
"read symbol-for-symbol." But at the end, the specific example is not
explained, so we speak in plain English.
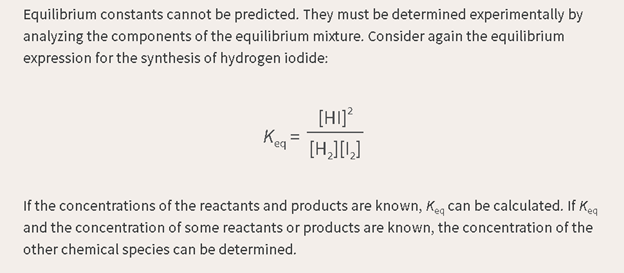
How we read it:
The
equilibrium constant is equal to the square of the concentration of hydrogen
iodide, divided by the concentration of hydrogen gas, and divided by the concentration
of iodide gas.
Reasoning:
Although the
second "divided by" is repetitious and not strictly necessary, it helps the
intelligibility of it.

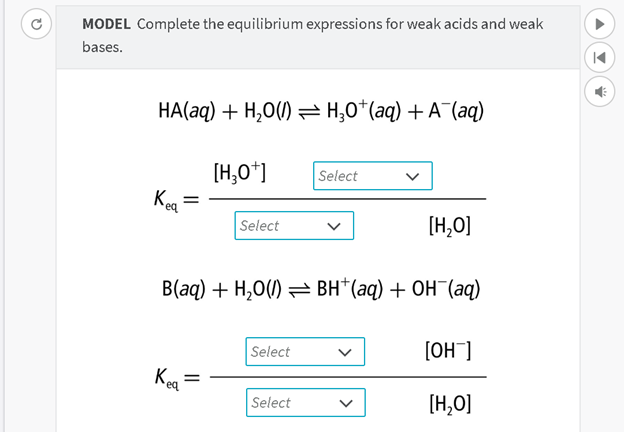
How we read it:
Aqueous H A and
liquid H 2 O is in equilibrium with aqueous H 3 O plus and aqueous A minus.
The
equilibrium constant is equal to the concentration of H 3 O plus times
something you select: Is it the concentration of H 2 O or the concentration of
A minus?
 How we read it:
How we read it:
Aqueous hydronium
ions and aqueous ammonia are in equilibrium with aqueous ammonium ions and
water.
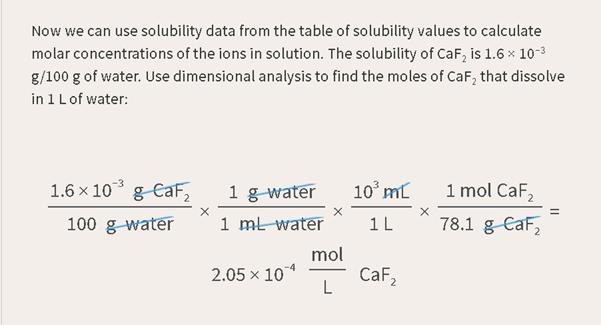
One point six
times ten to the negative 3 grams of calcium fluoride per 100 grams of water
times one gram of water per one milliliter of water, times ten to the third
milliliters per one liter, times one mole of calcium fluoride per seventy eight point one grams of calcium fluoride equals two
point zero five times ten to the minus four moles per liter of calcium
fluoride.

How we read it:
A unit of a
pink complex of aqueous cobalt two plus ions containing six units of water and
four units of aqueous chloride ions are in equilibrium with a unit of a blue
complex of aqueous cobalt two plus ion containing four units of chloride ions
and six water molecules.

How we read it:
The reaction
equation for the cobalt(II) chloride equilibrium
system is
A unit of
aqueous complex of cobalt two plus ions combined with six water molecules and
four units of aqueous chloride ions are in equilibrium with a unit of aqueous
complex of cobalt two plus ions combined with four chloride ions and six units
of liquid water.
The
equilibrium expression for this reaction is written as:
K e q equals
the concentration of the complex of cobalt two plus ion combined with four
units of chloride ions, divided by the concentration of the complex of cobalt
two plus ion combined with six units of water, divided by the concentration of
chloride ion to the fourth power.